What You Need to Know About the Covid-19 Vaccines
By Kathryn Saxton– March 30, 2022
What You Need to Know About the COVID-19 Vaccines
As countries around the world are experiencing another surge in COVID-19 cases due to the new Omicron variant, more pressure is being placed on the general public to make sure they are fully vaccinated against the virus. There are many different vaccines available in different places, and they all offer protection against the highly contagious strain now circulating. It is important for the general public to be aware of the different types of vaccines and how they work, as well as their availability and eligibility guidelines so that informed decisions can be made in order to protect our communities.
Types of Vaccines
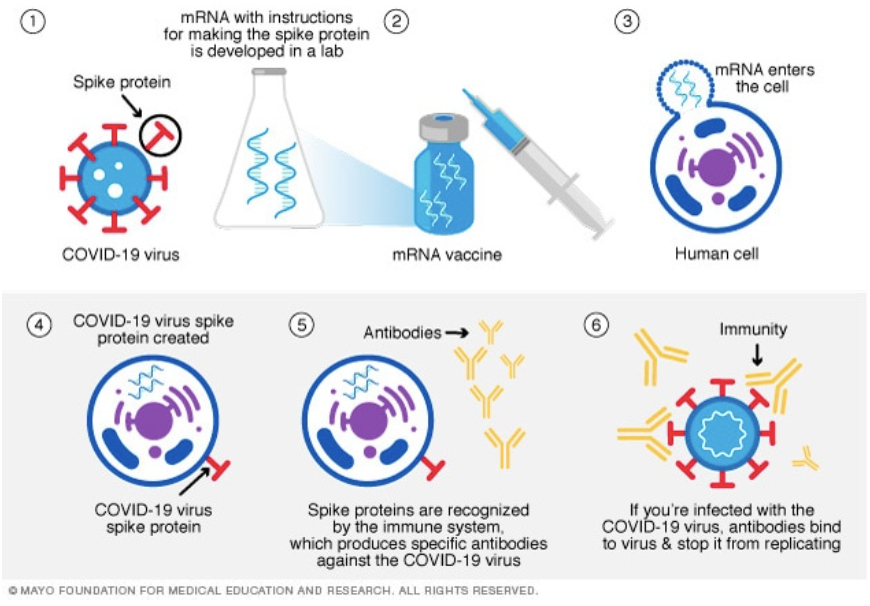
Currently, there are three main types of vaccines that are widely available. Perhaps the most discussed variety is the messenger RNA (mRNA) vaccines. RNA, or ribonucleic acid, is a form of genetic material that is read by ribosomes to produce proteins in cells. mRNA vaccines contain RNA that codes for the spike protein found on the outside of COVID-19 viruses. The cell that the mRNA enters starts to produce these proteins, which make their way to the cell surface. This triggers an immune response, and the body produces antibodies specific to the COVID-19 spike protein. If you are later infected with the actual coronavirus, the antibodies you produced will help fight off the virus. mRNA vaccines for other diseases have been in the works for some time, but the COVID-19 vaccine is the first of its kind to be approved and available to the public. A common concern about mRNA vaccines is their ability to infiltrate into your own genetic material. Luckily, the mRNA never enters the nucleus, where the cell’s genetic material is stored, and it does not remain in the cell for long before being broken down. Both the Pfizer-BioNTech and Moderna vaccines are mRNA vaccines.
Mayo Clinic, “Different types of COVID-19 vaccines: How they work”, https://www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/different-types-of-cOVID-19-vaccines/art-20506465, accessed Jan. 2022
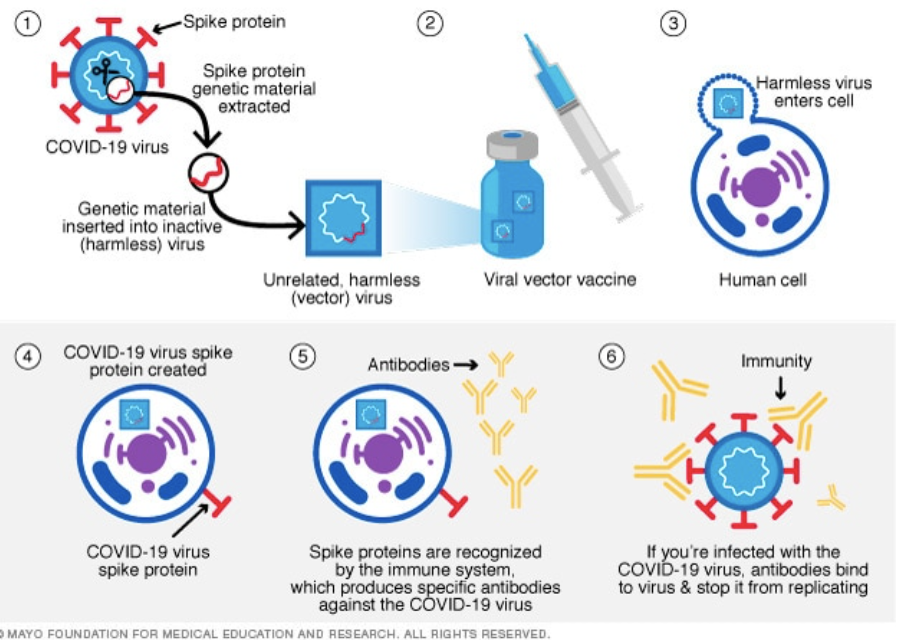
Another type of COVID-19 vaccine uses viral vectors. These vaccines are modified viruses that contain the genetic material of the COVID-19 virus and function in a similar way to the actual virus. Viruses survive by infecting cells and injecting their own genetic material into them so that more of the virus is produced. Similarly, viral vector vaccines result in cells receiving genetic information for the spike proteins from the vectors. As with the mRNA vaccines, the spike protein is produced by the cell, and antibodies are created. Viral vector vaccines are also used to protect against other diseases, such as Ebola. The most popular viral vector vaccines for COVID-19 are manufactured by Janssen/Johnson & Johnson and AstraZeneca.
Mayo Clinic, “Different types of COVID-19 vaccines: How they work”, https://www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/different-types-of-cOVID-19-vaccines/art-20506465, accessed Jan. 2022
The final variety of COVID-19 vaccines is known as protein subunit vaccines. These vaccines contain protein subunits of the spike protein found on the coronavirus. When these proteins enter the body, antibodies are generated in response. The vaccine created by Novavax is a protein subunit vaccine.
Mayo Clinic, “Different types of COVID-19 vaccines: How they work”, https://www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/different-types-of-cOVID-19-vaccines/art-20506465, accessed Jan. 2022
Vaccine Availability and Eligibility
In the United States, there are three vaccines available from different manufacturers: Pfizer-BioNTech (mRNA vaccine), Moderna (mRNA vaccine), and Janssen/Johnson & Johnson. The Pfizer vaccine is the only one of the three to have received full approval from the Food & Drug Administration (FDA), while the other two have emergency use authorization. The number of people eligible to receive a vaccine has increased over time, and anyone 5 and up can now be vaccinated against COVID-19. Children between the ages of 5 and 17 can only receive the Pfizer-BioNTech vaccine; adults 18 and up can have any of the three vaccines, although the Janssen/Johnson & Johnson is not preferred as it does not provide as much protection.
Dosage Schedule
The mRNA vaccines are two-dose series. For the Pfizer-BioNTech vaccine, this entails two shots with 21 days in between, whereas the Moderna series requires 28 days between doses. The Janssen/Johnson & Johnson vaccine series only consists of a single dose. Recipients of all three vaccines are considered fully vaccinated two weeks after the final dose of the primary series. The Centers for Disease Control and Prevention (CDC) is also recommending a booster shot to increase antibody levels. Moderna and Pfizer-BioNTech vaccines can be received 5 months after the last dose of the Moderna or Pfizer-BioNTech primary series or 2 months after the Janssen/Johnson & Johnson shot. One study has found that boosters can be mixed and matched, meaning that the brand of your booster does not have to match the brand of your primary series.
What To Expect After Receiving a COVID-19 Vaccine
Some people experience mild symptoms after receiving the vaccine, such as discomfort at the vaccination site, fatigue, or a headache. Luckily, adverse effects are very rare but the benefits are plentiful. Vaccines seem to lower transmission between individuals and help to reduce the severity of the virus if it is contracted. It is imperative that members of communities receive a full course of the vaccine, including a booster, as it protects the most vulnerable members of our society by helping the community reach herd immunity and preventing the virus from mutating further. Vaccine appointments can be made online, and many big brand pharmacies offer COVID vaccines on a walk-in basis.