The Dangers of Free Radicals and the Antioxidant Miracle
By Nathan Min – March 29, 2024
Imagine there exists a molecule so dangerous that it disfigures your DNA, promotes cancer, and causes Alzheimer’s disease. Even worse, imagine that these entities are present in all of us, produced by our own bodies. Well, you don’t have to imagine. These molecules—called free radicals—exist!
Free Radicals 101
What are free radicals? Put simply, free radicals are molecules with an unpaired electron. Examples include the “superoxide anion” (O2-), hydrogen peroxide (H2O2), and hydroxyl radical (OH). Why does this matter? In most “normal” molecules, electrons come in pairs. Meanwhile, because free radicals have unpaired electrons, they are very reactive and unstable. [6] The figure to the right shows how antioxidants donate electrons to free radicals.
Molecule Reactivity: An Aside
Why does having unpaired electrons make free radicals unstable? Recall that stable molecules tend to have electron pairs. Free radicals, like other molecules, want to achieve this stable configuration of having all their electrons paired. Thus, free radicals will often “steal” electrons from other molecules to pair them with their lone electrons, often causing molecular chaos. (Think of a single person trying to steal someone else’s romantic partner to become a pair!) More formally, it is said that free radicals “oxidize” other molecules.
Seemingly even worse, free radicals are unavoidable and are found in many places. In fact, many are generated through excessive exercise and normal biological processes such as enzymatic (ie. phagocytosis) and nonenzymatic reactions in your mitochondria. In addition, free radicals are common especially in urban/industrial environments, generated by cigarette smoke, ozone, radiation, some drugs and pesticides, industrial solvents, and other pollutants. [6]
Free radicals are particularly dangerous to life because they target biologically important macromolecules such as DNA/RNA, proteins, and lipids— functionally-diverse fats that make up our cell membranes. In more formal terms, an excess of free radicals in the body induces a state of “oxidative stress,” which has been associated with numerous life-threatening diseases, notably all inflammation-related diseases such as arthritis and lupus, AIDS, and neurodegenerative diseases, such as Alzheimer’s, Parkinson’s, and muscular dystrophy. [6]
Antioxidants: The Body’s Defense System
Luckily, however, free radicals are not “free” to wreak havoc on your body, thanks to a special class of molecules known as antioxidants! Antioxidants are your body’s natural warriors who defend you against the scourge of free radicals. How do they do it? One theory on their mechanism is the “chain-breaking method.” [6] Essentially, what this method entails is stopping the free radicals from stealing electrons by donating electrons to them. By giving electrons to free radicals, antioxidants turn unpaired electrons to paired electrons, thereby neutralizing the unstable nature of the free radical.
Antioxidants are also thought to “defend-in-depth,” with various layers of defense against free radicals. Some “1st layer” antioxidants seek to prevent radical formation in the first place while “3rd layer” antioxidants may repair and/or remove damaged proteins oxidized by radicals. [6]
Common Sources of Antioxidants
“So, if I need these antioxidants so much, where can I find them?” you may ask. The good news is that antioxidants, just like free radicals, are plentiful in our daily lives. Some important antioxidants are: selenium, manganese, glutathione, coenzyme Q10, lipoic acid, flavanoids, (poly)phenols, and phytoestrogens. [4]
Where can you find these antioxidants? (This is why your mom was always right— you should eat your veggies!) Here’s a list of some important sources.
Vitamin C: Broccoli, Brussels sprouts, cantaloupe, cauliflower, grapefruit, leafy greens (turnip, mustard, beet, collards), honeydew, kale, kiwi, lemon, orange, papaya, snow peas, strawberries, sweet potato, tomatoes, and bell peppers (all colors). [4]
Vitamin E: Almonds, avocado, Swiss chard, leafy greens (beet, mustard, turnip), peanuts, red peppers, spinach (boiled), and sunflower seeds. [4]
Carotenoids including beta-carotene and lycopene: Apricots, asparagus, beets, broccoli, cantaloupe, carrots, bell peppers, kale, mangos, turnip and collard greens, oranges, peaches, pink grapefruit, pumpkin, winter squash, spinach, sweet potato, tangerines, tomatoes, and watermelon. [4]
Selenium: Brazil nuts, fish, shellfish, beef, poultry, barley, brown rice. [4]
Zinc: Beef, poultry, oysters, shrimp, sesame seeds, pumpkin seeds, chickpeas, lentils, cashews, fortified cereals. [4]
Phenolic compounds: Quercetin (apples, red wine, onions), catechins (tea, cocoa, berries), resveratrol (red and white wine, grapes, peanuts, berries), coumaric acid (spices, berries), anthocyanins (blueberries, strawberries). [4]
However, it’s important to note that while all of these compounds are antioxidants, they are not the same; different antioxidants have different effects, and may be helpful in some circumstances and harmful in others. Furthermore, despite what nutrition companies may tell you, antioxidant supplements have not been proven by research to be as useful as commercials and advertisements claim moderate benefits, if any. Most research findings regarding the ability of supplements like vitamin E and other antioxidants to help reduce changes of heart disease and cancer remain inconclusive. For example, in the Physicians’ Health Study, patients taking beta-carotene supplements had roughly the same rates of cancer as compared with those who took the placebo. [4]
All throughout your body, then, the epic war between free radicals and antioxidants is raging like in a saga of Star Wars. Normally, antioxidants (the Jedi) are plenty enough to keep free radicals (the Sith) in check. However, a lack of antioxidants can lead to oxidative stress (a disturbance in the force), as discussed earlier, causing a variety of diseases.
Case Study: Cancer
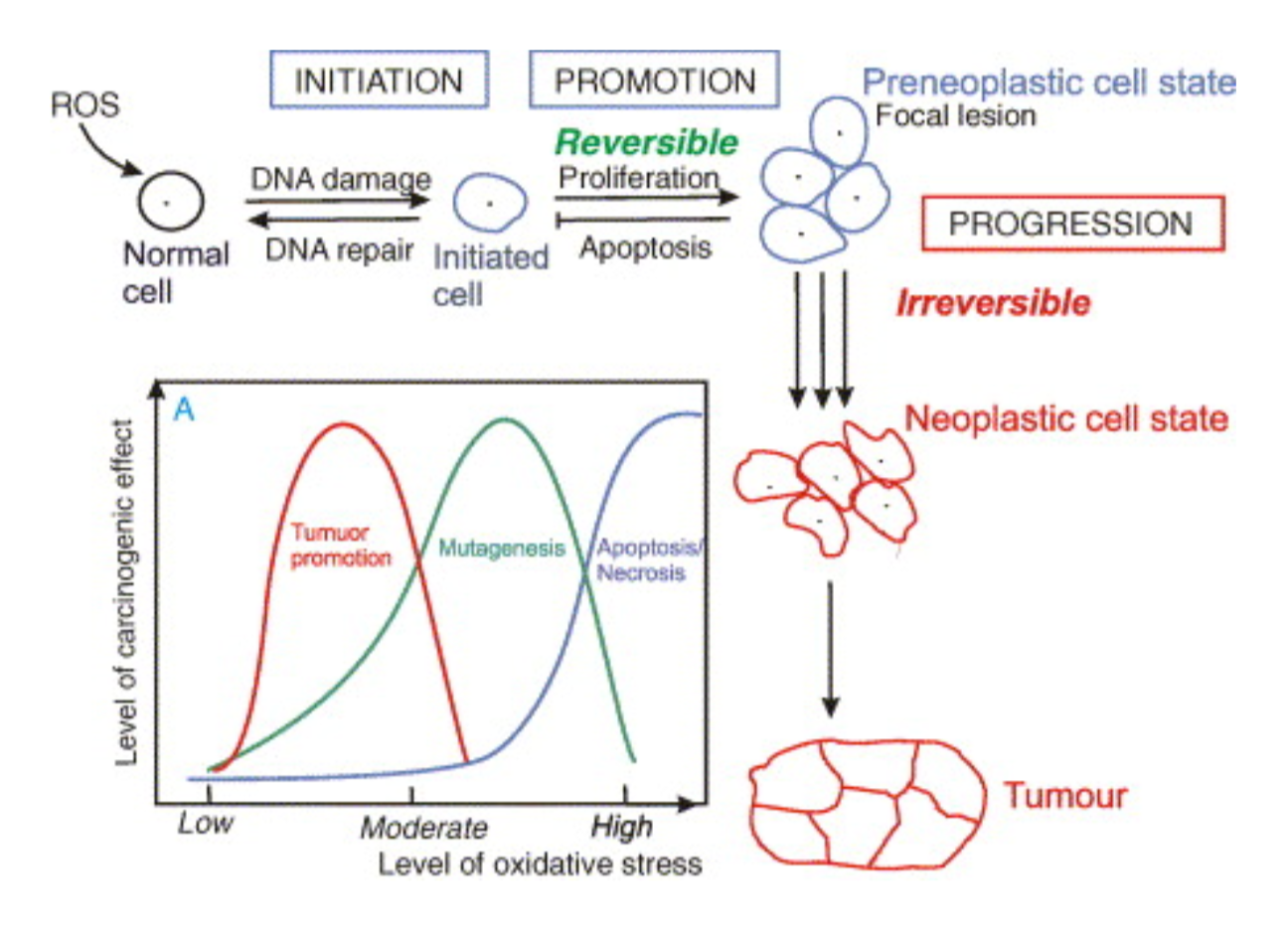
Cancer affects roughly 403 people for every 100,000 people in the US, and is often rooted in free radical damage. [1] For example, through damaging DNA, free radicals increase rates of mutation and potential for transformation (becoming cancerous) in cells, causing cancer. Meanwhile, antioxidants such as Vitamin C, Vitamin E, and B-carotene help counter radicals’ carcinogenic effects. They have many effects, such as upregulating the immune system and DNA repair. [6]
This figure shows how reactive oxygen species (ROS)—a type of free radical—help create “preneoplastic” cells. [3, 5, 7] This means that ROS create mutated cells that are more likely to form malignant tumors that spread around the body like an infection (cancer). We can also see the “stages” of cancer development that occur as ROS levels and oxidative stress increase.
Case Study: Cardiovascular Disease
Another example is cardiovascular disease, which was responsible for 1 in 5 deaths in the US in 2021. [2] Low-density lipoprotein (LDL) oxidized by free radicals contributes to atherosclerosis plaques and also damages endothelial cells, promoting cardiovascular disease. Meanwhile, antioxidants B-carotene and Vitamin E help counter against such effects. [6]
As we can see, antioxidants are vital in preventing the detrimental effects of free radicals in the body. So, if you ever feel lonely in these tough times, remember—you’ve got millions of microscopic molecules rooting for you!
Nathan Min
B.S. Molecular, Cell, & Developmental Biology – Class of 2027